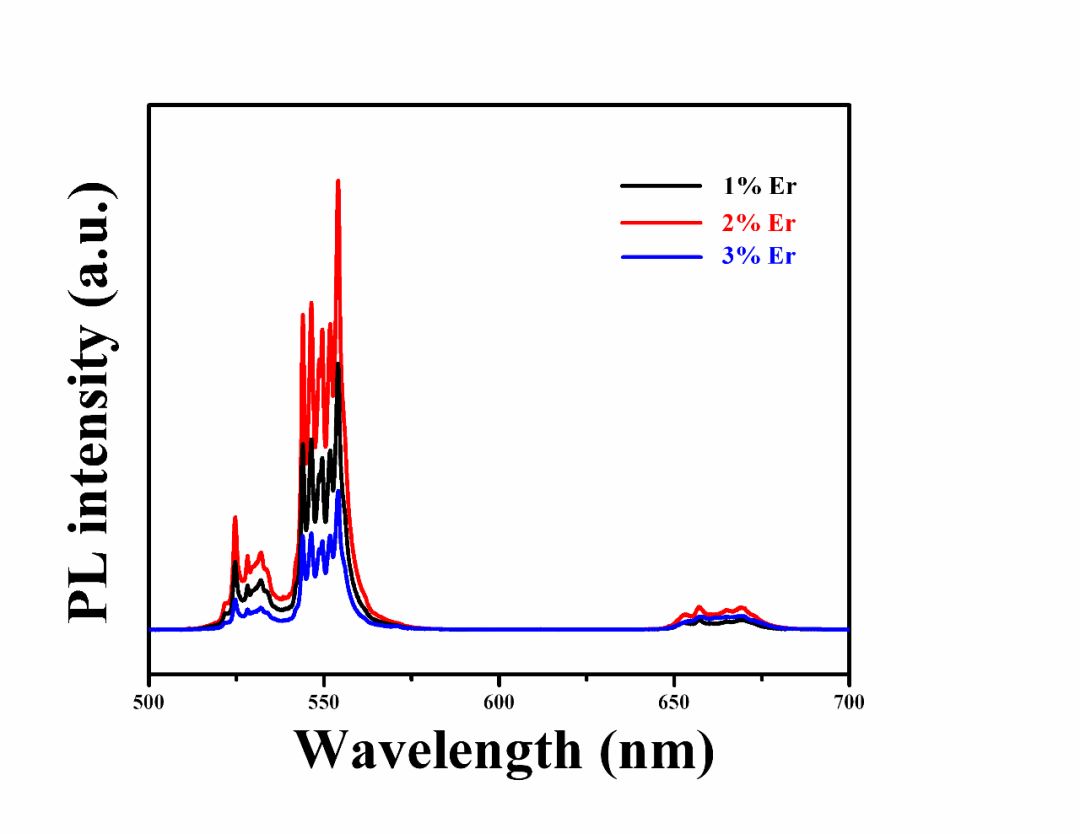
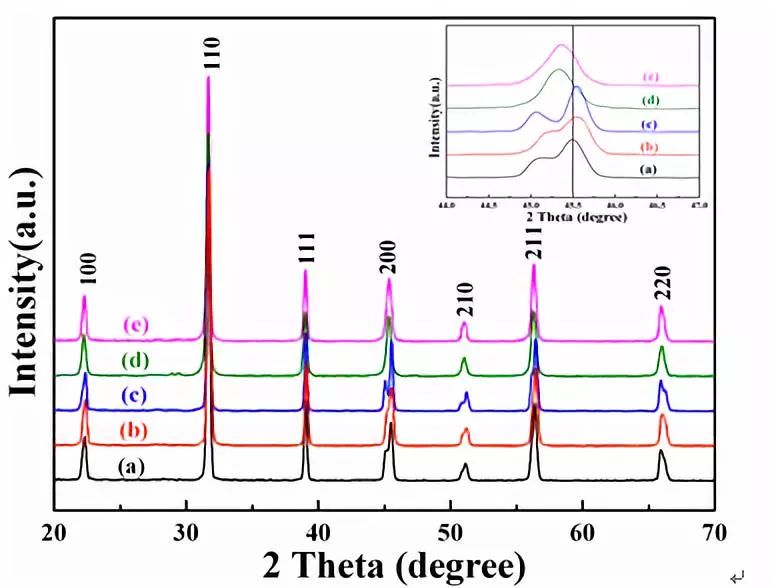
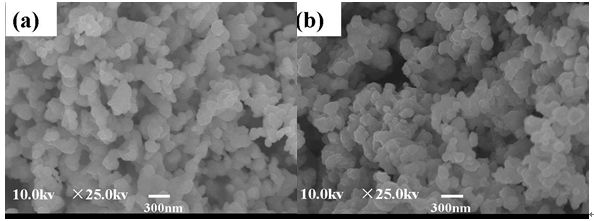
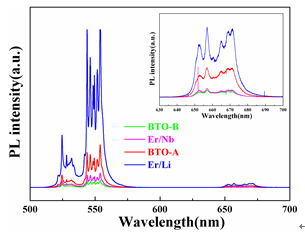
The powders with different lattice sites of Er3+ instead of BaTiO3 were prepared by the high-temperature solid-state reaction method and the Ba/Ti ratio was adjusted. The results show that Er3+ doping with different positions will lead to significant changes in the symmetry and luminous intensity of the crystal structure. According to the principle of charge compensation, Li+ and Nb5+ are co-doped at the Ba and Ti sites, respectively, and their effects on the crystal structure, morphology and up-conversion luminescence properties are studied. With the use of Er3+, Li+ and Er3+, Nb5+ co-doped, the upconversion luminescence intensity of the powder was significantly improved. It is worth noting that the intensity of the Er3+, Li+ co-doped Ba-bearing samples is higher than that of the Er3+ and Nb5+ co-doped Ti-bearing samples, indicating that the enhancement of the two strengths is attributed to different mechanisms. The improvement of the intensity of the Er3+ and Nb5+ co-doped samples is mainly attributed to the principle of charge compensation, while the Er3+ and Li+ co-doping results from the reduction of the symmetry of the crystal field and the compensation of the charge. introduction In recent years, rare earth-doped perovskite structure materials have been widely used in the fields of up-conversion luminescence, fluorescent labeling, display and structure probes, and have received great attention from researchers [1-5]. Among rare earth ions, the rare earth Er3+ has abundant 4f electronic energy levels, the emission wavelength at 1550 nm is exactly at the lowest loss of optical fiber for optical fiber communication and has strong excitation state absorption at 800 nm and 980 nm laser excitation. It is widely used as an activator ion for up-conversion luminescent materials [6-8]. The perovskite structure material has good structure and chemical stability, a wide band gap, and a large degree of adjustment to the filled ionic radius, and is therefore very suitable as an up-conversion luminescent matrix material. Among them, in addition to excellent ferroelectric properties, BaTiO3 (BTO) has excellent electro-optic properties such as high electro-optic coefficient (820 pm·V-1, LiNbO3 only 30.8 pm·V-1) and low half-wave voltage. (310 V, LiNbO3 is 2940 V), low waveguide loss (≤4±2 dB·cm-1) and high Er ion solubility [9]. It is worth pointing out that the radius of Er3+ is between Ba2+ and Ti4+. Er3+ can be doped with either Ba(A) or Ti(B), and its doping position can be controlled by the ratio of Ba/Ti [5, 15]. This makes Er3+ incorporation into BTO not only structurally tunable but also has superior optical and electrical properties. Therefore, in recent years, researchers have used a variety of methods to study the influencing factors of Er3+-doped BTO luminescent materials, including controlling temperature and electric field, co-doping ions, controlling the occupancy of Er3+, and controlling the change of matrix phase structure. Wait [10-16]. Among these regulatory methods, ion co-doping is considered to be the most efficient method. At present, there are many reports on the upconversion emission intensity by ion co-doping, such as the use of Er3+/Yb3+ co-doped BTO lattices by MA Meneses-Nava, which improves the emission intensity through the energy transfer mechanism [17]; Q. Sun et al. use Er3+ The co-doped BTO lattice of Li/Li+ reduces the symmetry of the crystal field and enhances the upconversion emission [12]. However, it has rarely been reported that co-doping ions increase the luminescence intensity at different positions of BTO. In addition, co-doping in different positions of BTO not only compensates the charge, but also changes the symmetry of the crystal field around Er3+. Both of them have a certain influence on the upconversion luminescence, but at present no one has studied the principle of charge compensation and the influence of the symmetry of the crystal field on the luminescence of the upconversion. In this work, Er3+/Li+ co-doped BTO A sites and Er3+/Nb5+ co-doped BTO B sites were prepared by high temperature solid-state reaction. The effects of ion codoping on the structure, morphology and luminescence of the samples were studied and analyzed. The mechanism for enhancing the up-conversion luminescence performance. 1. Experiment 1.1 Sample preparation Using analytically pure BaCO3, SrCO3, Li2CO3, TiO2, Nb2O5 and Er2O3 as raw materials, Er3+-doped BaTiO3 A-position (BTO-A) and B-position (BTO-B) powders were prepared by high temperature solid-state method and were The corresponding lattice sites are co-doped with Li+ and Nb5+. According to the charge compensation mechanism, Er3+, Li+ replaces the A and Er3+ simultaneously, and Nb5+ simultaneously replaces the B position. The chemical formulas are ErxBa1-2xLixTiO3 (Er/Li) and BaErxTi1-2xNbxO3 (Er/Nb), respectively. The raw materials were weighed strictly in accordance with the stoichiometric ratio, and each was placed in a polyurethane ball mill tank and deionized water ball mill was added for 24 hours. Take out and dry, and burn in the muffle furnace at 1100°C for 8 hours to obtain the final powder sample. 1.2 Characterization X'Pert Pro MPD X-ray diffraction (XRD) was used to analyze the crystal phase composition and crystal structure of the sample. The surface morphology of the sample was observed with a Hitachi Model S-4800 scanning electron microscope. Hitachi F-4500 fluorescence spectrometer was used to analyze the upconversion luminescence of the sample. The excitation light source uses a 980 nm laser diode with a maximum excitation power of 150 mW. 2 Results and Discussion 2.1 Effect of Er3+ Concentration on Upconversion Luminescence Figure 1 shows the PL spectra of different doping concentrations of BTO-A powder excited by a 980 nm laser. The emission at 523 nm, 563 nm, and 656 nm in the figure is attributed to transitions of three energy levels of Er3+, which are 2H11/2/4S3/2→4I15/2 and 4F9/2→4I15/2, respectively. With the increase of Er3+ concentration from 1% mol to 2% mol, the upconversion luminescence intensity increases, but when the Er3+ concentration is 3% mol, the luminescence intensity decreases. This similar phenomenon has also been published by Q.Sun et al. This is mainly due to the fact that as the concentration of Er3+ increases, the interaction between ions increases, causing cross-relaxation, eventually leading to concentration quenching [5]. In order to avoid concentration quenching, Er3+ concentration was fixed at 1% mol in the following experiment. Fig.1 Up-conversion emission spectra of different Er3+ concentrations in BTO-A powders 2.2 Effect of Ion Doping on Crystalline Structure and Morphology of Samples FIG. 2 shows the XRD patterns of different lattice positions and ion co-doping of Er3+-doped BTO. It can be seen that after the doping, the structure shows a single perovskite structure and no heterophase appears, indicating that the doped ions have entered the perovskite crystal lattice. The internal drawing is an enlarged view of the (200) crystal plane. It can be seen that the (200) diffraction peaks of both the BTO-A and BTO-B samples are slightly shifted relative to the undoped BTO sample. This is mainly due to the fact that the Er3+ radius is between Ba2+ and Ti4+. In place of the A and B sites, the lattices are compressed and expanded. This phenomenon is consistent with that published by Y. Zhang et al. [5], further illustrating that Er occupies different lattice positions. It is worth noting that the Er3+ valence state is between Ba2+ and Ti4+. When occupying the A and B sites, respectively, it will inevitably cause charge imbalance. Therefore, Li+ and Nb5+ codoping are used here, mainly to compensate for the lack of charge. However, from the XRD pattern, it was found that ion co-doping also caused a shift in the tetragonal structure of the crystal. After calculation, the c/a values ​​of BTO, BTO-A, BTO-B, Er/Li, and Er/Nb samples were 1.053, 1.093, 1.015, 1.125, and 1.021. It can be seen that the co-doping of Nb5+ does not substantially change the symmetry of the crystal structure, whereas the structural symmetry of the Li+ co-doped sample is significantly reduced. Changes in the symmetry of the structure will inevitably lead to changes in the symmetry of the crystal field around Er3+, which in turn will affect the luminous intensity of the sample. Fig.2 XRD patterns of different lattice positions and ion co-doping of Er3+ doped BTO (a) pure BTO; (b) BTO-A; (c) Er/Li; (d) BTO-B; (e) Er/Nb Figure 3 shows scanning electron microscope (SEM) images of BTO-A and BTO-B samples. It can be seen that the grain morphology of Er3+ doped at different positions is approximately the same. Grains have a small amount of agglomeration phenomenon, the grain size is about 300-500nm. With the co-doping of Li+ and Nb5+, the morphology of the sample did not change significantly. Fig. 3 SEM image of Er3+-doped BTO samples with different lattice positions; (a) BTO-A, (b) BTO-B Effect of Ion Codoping on Luminescence Properties of Samples Fig. 4 is the upconversion luminescence spectrum of Er3+-doped BTO measured at room temperature with 980nm laser excitation and ion co-doped. It can be seen that the spectral splitting is more obvious, indicating that there is a strong crystal field around Er3+. Compared with the BTO-B sample, the BTO-A sample has a stronger emission intensity, which is consistent with the results reported by Zhang Yang et al. This may be mainly caused by the following two reasons: On the one hand, when Er3+ is doped with B, the oxygen vacancies due to the non-conservation of charge cause its luminescence quenching [5]; on the other hand, the value of c/a Analysis, BTO-A has lower structural symmetry than BTO-B sample, which means that the BTO-A sample has a lower symmetry around the Er3+ crystal, which leads to a strong emission intensity of the BTO-A sample. In addition, with the co-doping of Li+ and Nb5+, the luminescence intensity of the two increased by about 1 and 5 times, respectively. This is mainly because when Er3+ replaces the A and B sites, respectively, due to the imbalance of the charge, there will be Ba vacancies and oxygen vacancy defects, respectively [18]. However, Li+ and Nb5+ are co-doped in the A and B sites, respectively, to maintain the charge conservation, reduce the generation of defects, and thus increase the luminous intensity. It is worth noting that the luminescence intensity of Er/Li is much more significant than that of Er/Nb sample. Therefore, it can be inferred that in addition to the charge compensation mechanism in Er/Li sample, there is definitely another mechanism that mainly enhances the emission intensity of the sample. From the analysis of the sample structure in Figure 2, it can be seen that after the co-doping of Er3+ and Li+, the structural symmetry of the sample is significantly reduced, which means that the crystal field around the Er3+ in the Er/Li sample has a lower symmetry. Therefore, the probability of ff transition of Er3+ in the sample increases, which significantly increases the emission intensity of the sample. In the Er/Nb sample, the codoping of Nb5+ has little effect on the crystal structure, and the crystal field around Er3+ does not change substantially, so the luminescence intensity of the sample is less affected. Figure 4 The different lattice positions of Er3+-doped BTO and the co-doped upconversion luminescence spectra measured at room temperature using 980 nm laser excitation. in conclusion The powders with different positions of Er3+-doped BTO were prepared by the solid-state method, and Li+ and Nb5+ were co-doped at the A and B sites, respectively, so that the upconversion luminescence properties of the powders were enhanced to varying degrees. The up-conversion luminescence intensity of the Er/Li co-doped sample was enhanced by about 5 times, while the up-conversion luminescence intensity of the Er/Nb co-doped sample was only increased by about 1 time. The analysis shows that the co-doping of Er3+ and Li+ not only compensates for the charge mismatch, but also changes the symmetry of the crystal field. The up-conversion luminescence enhancement is mainly attributed to the decrease of the crystal field symmetry and the charge compensation mechanism. Er3+, Nb5+ Doping basically has no effect on the crystal field, so its up-conversion enhancement is mainly due to the charge compensation mechanism. 1500 Puffs Vape,E-Cigarette 1500 Puffs,Disposable Vape Pen E-Cigarette,All-In-One Electronic Cigarette Guangzhou Yunge Tianhong Electronic Technology Co., Ltd , https://www.e-cigarettesfactory.com